Glyceraldehyde-3-Phosphate Dehydrogenase Assay
Method: The initial reaction velocity is measured as an increase in absorption at 340 nm resulting from the reduction of NAD. The assay system is a modification of the procedures described by Krebs (1955) and Velick (1955). One unit causes an initial rate of reduction of one micromole of NAD per minute at 25°C and pH 8.5 under the specified conditions.
Reagents
- 0.015 M Sodium pyrophosphate buffer, pH 8.5 containing 0.03 M sodium arsenate
- 7.5 mM NAD. Note: NAD may vary in salt form and degree of hydration. Care should be exercised to use an analytical grade and the correct molecular weight.
- 0.015 M DL-glyceraldehyde-3-phosphate (7.5 mM D-glyceraldehyde-3-phosphate). See note below.
- 0.1 M Dithiothreitol (DTT)
Enzyme
Immediately prior to use, dilute in pyrophosphate/arsenate buffer to a concentration of 10 - 30 micrograms/ml.
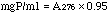
Procedure
Adjust spectrophotometer to 340 nm and 25°C.
Pipette into each cuvette as follows:
| Pyrophosphate/arsenate buffer | 2.6 ml |
| 7.5 mM NAD | 0.1 ml |
| 0.1 M DTT | 0.1 ml |
| Enzyme | 0.1 ml |
Incubate in spectrophotometer at 25°C for 3 - 5 minutes to achieve temperature equilibrium and establish blank rate, if any. At zero time, add 0.1 ml of 0.015 M DL-glyceraldehyde-3-phosphate and record A340 for 3 - 5 minutes. Determine ΔA340/minute from the initial linear portion of the curve.
Calculation
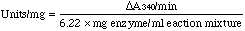
Note: D-glyceraldehyde-3-phosphate can be prepared from its DL-glyceraldehyde-3-phosphate diethylacetal derivatives as follows:
- Suspend 1.5 grams of Dowex-50 hydrogen form resin in 6 ml of reagent grade water in a test tube.
- Add 100 mg of DL-glyceraldehyde-3-phosphate diethylacetal, barium salt, and mix thoroughly.
- Place the test tube in a boiling water bath for 3 - 5 minutes with occasional mixing. Cool quickly in an ice bath.
- Centrifuge and decant the supernatant which contains the free acid form of DL-glyceraldehyde-3-phosphate.
- Wash the resin several times with 2 ml aliquots of water to complete the extraction. Combine the supernatants and determine the D-glyceraldehyde-3-phosphate concentration. If the hydrolysis and extraction are complete the pooled supernatants should contain approximately 100 micromoles of the D-isomer.
The concentration of the D-isomer can be measured by the above assay utilizing an excess of enzyme.
Up: Worthington Enzyme Manual

 Place Order
Place Order