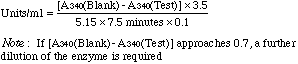NADase (DPNase) Assay
Method: Cyanide reacts with the quaternary nitrogen form of NAD to form an addition product with an absorbance maximum at 340 nm (Colowick et al. 1951). No such reaction occurs with nicotinamide or adenosine-diphosphate-ribose. Therefore, the reaction between cyanide and NAD before and after incubation with NADase will indicate the activity of the enzyme (Kaplan 1955; Nason et al. 1951). One unit will cleave one micromole of NAD per minute at 37°C and pH 7.5. Note: The original method used a unit which was equivalent to the splitting of 0.01 micromole of NAD in 0.5 ml in 7.5 minutes.
Reagents
Enzyme Reconstitute vial contents with one milliliter reagent grade water. Immediately prior to use dilute further to a concentration of 0.10-0.50 mg/ml. Procedure Pipette into test tubes as follows:
Incubate at 37°C for 3-5 minutes to achieve temperature equilibration. At zero time add 0.1 ml appropriately diluted enzyme to assay and blank tube. Incubate for exactly 7.5 minutes at 37°C and add 3.0 ml of 1.0 M KCN to assay tube. Cool to room temperature and read A340 of blank and assay tubes. Calculation |

 Place Order
Place Order