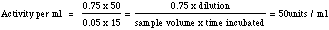Neutral Protease (Dispase) AssayMethod The reaction velocity is determined in a peroxidase coupled system by measuring the increase in A436 resulting from the oxidation of D-alanine. One unit oxidizes one micromole of D-alanine per min. at 37?C and pH 8.3 under the specified conditions. Reagents The nature of this enzyme requires the substrate to be prepared accurately. Take care to follow the instructions below. 1M Tris Buffer-Substrate (2% casein in 0.05M Tris buffer): Dissolve 1.0gm casein (USB grade or equivalent) in 40.0ml reagent grade water by stirring for approximately twenty minutes. Add 1.0ml of 1N NaOH and 2.5ml of 1.0M Tris. Allow substrate to stir until casein has gone into solution. pH to 7.8 with dilute phosphoric acid and continue stirring for 5 minutes. Bring to a final volume of 50.0ml with reagent grade water. Prior to pipetting substrate make sure substrate is homogeneous throughout and casein has not fallen out of solution. If casein appears to have fallen out of solution, do not use substrate for assay. Enzyme activator - 0.01 M CaCl2: Dissolve 147 mg CaCl2 in 100 ml reagent grade water. When not used keep in refrigerator. 5% TCA: Dissolve 5 gms TCA in 100 ml reagent grade water. Keep at room temperature. Tyrosine standard: Prepare 10 ml at a concentration of 10 μ moles/ml in reagent grade water. (181μg tyrosine = 1μmole). Weigh 18.1 mg tyrosine and dissolve in 10ml reagent grade water by stirring to initial boil. Remove from heat and cool to approximately 40°C for assay. 0.5 M NaOH: Dissolve 1 gm. in 50 ml reagent grade water, or make a dilution from 1M or 10M NaOH. Folin & Ciocalteus Phenol Reagent, use neat Enzyme Dissolve in deionized water, at a concentration of 5 mg/ml, and prepare 1:50 dilution. Use 25μl and 50μl in duplicate. Procedure To clear glass tubes add 1.0 ml buffer-substrate, 20 µL of 0.01M CaCl2, using a Pasteur pipette, and two levels of the enzyme dilution: 25μl and 50μl, each level in duplicate. Also include two tubes for blank, with no enzyme, and four tubes for tyrosine standard with 1ml of buffer substrate, and 25 μl, 50 µl, 75 μl, and 100 μl of tyrosine standard solution. Incubate for 15 minutes at 37°C in a water bath. Stop reaction by adding 2 ml 5% TCA. Mix each tube, then centrifuge at 2000 rpm for 10 minutes using a bench top Sorvall, or equivalent. From each tube withdraw (quantitatively) 1ml supernatant and add to marked clean glass tubes. Then add 2.0ml 0.5M NaOH to each tube. Mix, then add 0.1ml Folin-Ciacalteu reagent and read at A550 after 10 minutes. Plot A550 vs. μmoles tyrosine. Draw best fit line and select a sample reading that falls within the linear portion of the line and calculate as listed below. Calculation
|

 Place Order
Place Order