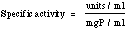Ribonuclease T1 AssayMethod: The method used at Worthington is essentially that of Egami et al. (1964) based upon the release of acid soluble oligonucleotides following the digestion of yeast RNA. One unit releases acid soluble oligonucleotides equivalent to one A260 in the reaction mixture at 37°C and pH 7.5 for 15 minutes. Reagents 0.2 M Tris•HCl buffer, pH 7.5: Dissolve 2.4 gms Tris (MW 121.14) in 95 ml reagent grade water and adjust pH to 7.5. Bring to a final volume of 100 ml with reagent grade water. 0.02 M EDTA: Dissolve 745 mg into a final volume of 90 ml reagent grade water. Adjust pH to 7.5 with NaOH and bring to a final volume of 100ml. RNA Soluton: Prepare a fresh solution of 12 mg/ml RNA (Roche catalog #109223; if not available consult supervisor) by suspending in approximately 80% of final volume of reagent grade water. Slowly add 1N NaOH so pH approaches 8.0. pH may drop as RNA dissolves. Repeat pH adjustment until RNA is completely dissolved and pH remains steady at 8.0 ± 0.1. QS to final volume wth reagent grade water. Check the A260 of the RNA using a 350x dilution to ensure the RNA is at or near 12 mg/ml. The A260 of a 350x dilution should be 0.85 ±0.05. Note: A260 (dilution factor) (40 μg/ml) = mg/ml RNA Perchloric acid/uranyl acetate solution: Dilute 22ml 70% perchloric acid to 100ml with reagent grade water. Add 750mg uranyl acetate and dissolve. Enzyme For protein determination, read A280, diluting as needed in reagent grade water and reading vs a 2.8M ammonium sulfate/reagent grade water blank. Mg protein/ml = A280 x 0.526 x dilution Also check using A260/A280 graph by E. Adams [Biochem Z. 310, 384 (1942)] and use the lower number. Immediately prior to use, dilute enzyme in 0.2 M Tris•HCl buffer pH 7.5 to several dilutions ranging between 1:1,000 to 1:10,000. Procedure Buffer mixture: Prepare according to number of reactions, at the following proportion: 0.25ml 0.2M Tris, pH 7.5 0.25 ml RNA solution For 40 sample tubes, prepare buffer mix of: 10ml 0.2M Tris, pH 7.5 10 ml RNA solution Pipette into tubes as follows: 0.90 ml reaction mix to each reaction tube. Include at least 3 tubes with reagent grade water instead of sample as blanks. Incubate tubes in a 37°C water bath for 5 minutes. At timed intervals add 0.1 ml diluted enzyme sample (or diluent for blanks) to all tubes. Incubate exactly 15 minutes then stop reaction by ading 0.25ml perchloric acid solution. Centrifuge at 2500 RPM for 5 minutes. Withdraw 0.20ml clear supenatant to tubes containing 4.80ml reagent grade water. Mix and read A260 versus water. Calculation 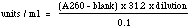
Note: this assay should be run with dilutions yielding results of A260 sample between 0.25 and 0.15 after subtracting blank. |

 Place Order
Place Order