Introduction to EnzymesThe following has been excerpted from a very popular Worthington publication which was originally published in 1972 as the Manual of Clinical Enzyme Measurements. While some of the presentation may seem somewhat dated, the basic concepts are still helpful for researchers who must use enzymes but who have little background in enzymology. Chemical Nature of EnzymesAll known enzymes are proteins. They are high molecular weight compounds made up principally of chains of amino acids linked together by peptide bonds. See Figure 1.
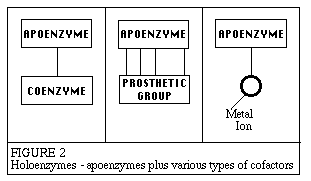
Enzymes can be denatured and precipitated with salts, solvents and other reagents. They have molecular weights ranging from 10,000 to 2,000,000. Many enzymes require the presence of other compounds - cofactors - before their catalytic activity can be exerted. This entire active complex is referred to as the holoenzyme; i.e., apoenzyme (protein portion) plus the cofactor (coenzyme, prosthetic group or metal-ion-activator) is called the holoenzyme.
Apoenzyme + Cofactor = Holoenzyme According to Holum, the cofactor may be: 1. A coenzyme - a non-protein organic substance which is dialyzable, thermostable and loosely attached to the protein part. 2. A prosthetic group - an organic substance which is dialyzable and thermostable which is firmly attached to the protein or apoenzyme portion. 3. A metal-ion-activator - these include K+, Fe++, Fe+++, Cu++, Co++, Zn++, Mn++, Mg++, Ca++, and Mo+++. Next: Specificity of Enzymes |

 Place Order
Place Order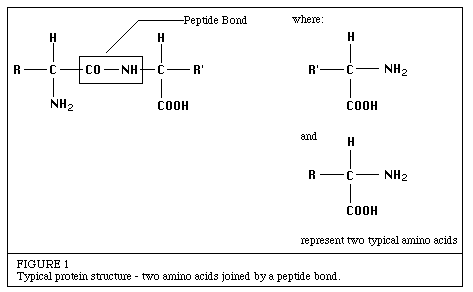
 PDF version of Introduction to Enzymes
PDF version of Introduction to Enzymes