Introduction to EnzymesThe following has been excerpted from a very popular Worthington publication which was originally published in 1972 as the Manual of Clinical Enzyme Measurements. While some of the presentation may seem somewhat dated, the basic concepts are still helpful for researchers who must use enzymes but who have little background in enzymology. Enzyme Kinetics: Energy LevelsChemists have known for almost a century that for most chemical reactions to proceed, some form of energy is needed. They have termed this quantity of energy, "the energy of activation." It is the magnitude of the activation energy which determines just how fast the reaction will proceed. It is believed that enzymes lower the activation energy for the reaction they are catalyzing. Figure 3 illustrates this concept. 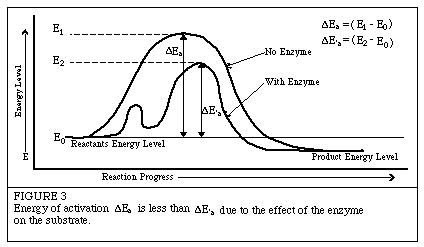 The enzyme is thought to reduce the "path" of the reaction. This shortened path would require less energy for each molecule of substrate converted to product. Given a total amount of available energy, more molecules of substrate would be converted when the enzyme is present (the shortened "path") than when it is absent. Hence, the reaction is said to go faster in a given period of time. Next: The Enzyme Substrate Complex |

 Place Order
Place Order PDF version of Introduction to Enzymes
PDF version of Introduction to Enzymes