Introduction to EnzymesThe following has been excerpted from a very popular Worthington publication which was originally published in 1972 as the Manual of Clinical Enzyme Measurements. While some of the presentation may seem somewhat dated, the basic concepts are still helpful for researchers who must use enzymes but who have little background in enzymology. Effects of pHEnzymes are affected by changes in pH. The most favorable pH value - the point where the enzyme is most active - is known as the optimum pH. This is graphically illustrated in Figure 14. 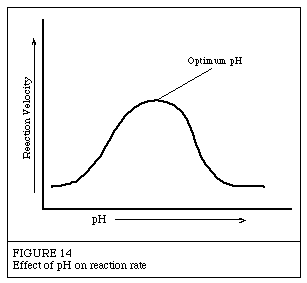 Extremely high or low pH values generally result in complete loss of activity for most enzymes. pH is also a factor in the stability of enzymes. As with activity, for each enzyme there is also a region of pH optimal stability. The optimum pH value will vary greatly from one enzyme to another, as Table II shows:
In addition to temperature and pH there are other factors, such as ionic strength, which can affect the enzymatic reaction. Each of these physical and chemical parameters must be considered and optimized in order for an enzymatic reaction to be accurate and reproducible. Next: References |

 Place Order
Place Order PDF version of Introduction to Enzymes
PDF version of Introduction to Enzymes