
Worthington Tissue Dissociation Guide
Optimization Techniques: Cell Quantitation
It is important to quantitate the results of each dissociation step in order to effectively evaluate each procedure. The use of a cell counting chamber (hemocytometer) for yield quantitation and the use of trypan blue for viability quantitation are recommended. The use of a hemocytometer for cell yield quantitation is outlined; however, newcomers to this procedure can refer to more detailed discussions (see Freshney, Culture of Animal Cells, page 227).
Required Supplies:
- Improved Neubauer Hemocytometer
- Cell Compatible Media or BSS
- Pasteur Pipet or Micropipettor
- Microscope (10X)
- Counter
Procedure:
- Carefully clean the counting chamber surface and the coverslip of the hemocytometer with 70% isopropanol and allow to air dry. Be careful not to scratch these surfaces.
- Wet the sides of the coverslip with reagent grade water and align the coverslip over the counting chamber.
- Take a well mixed 20-50 µl aliquot of the dissociated cell suspension using either a Pasteur pipet or a micropipettor only drawing the cells into the tip. Immediately transfer the cell suspension to the counting chamber by placing the tip of the pipet at the edge of the chamber and allowing the chamber to fill completely via capillary action. Do not over- or underfill the chamber.
- Repeat this procedure using another aliquot sample for the second chamber on the opposite side of the hemocytometer.
- Place the hemocytometer on the microscope stage and, using the
 10X objective, focus on the counting chamber grid lines. Adjust the contrast as needed to clearly see both the grid and the dispersed cells.
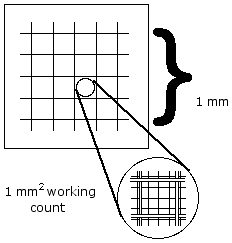
10X objective, focus on the counting chamber grid lines. Adjust the contrast as needed to clearly see both the grid and the dispersed cells. - Adjust the field area by slowly moving the slide to obtain a central grid bounded by three lines on all sides (see figure below). Count the total number of cells present in this 1 mm2 area including those cells which are on the top and left borders and excluding those on the right and bottom borders.
- For accuracy count at least 100-500 cells. Depending upon yield and density more or fewer areas may be counted.
- Repeat the count for the second chamber. If no second chamber exists, the slide should be cleaned and the process repeated.
Calculation:
C = Ñ x 104 where C = cells per milliliter
Ñ = average of cells counted
104 = volume conversion factor for 1 mm2
Total Yield = C x V where V = total vlaue of cells (ml)
Example:
Count1 = 183 cells/mm2
Count2 = 175 cells/mm2
Volume of Cells = 55 ml
| Average cells counted = | Count1 + Count2 |
| | 2 |
| = | 185 + 175 | |
| | 2 | |
| | | |
| = | 178.5 |
C = 178.5 x 10
4 = 1,785,000 cells/ml
Total yield = C x V = 1785,000 x 55 = 98,175,000 cells
Note: For best results the cell density should be at least 105 cells per milliliter. Common errors occur by improper mixing of the cell suspension prior to sampling and/or by allowing the cells to settle in the pipet prior to loading the hemocytometer counting chamber. Avoid the counting of multiple cell aggregates; the presence of aggregates indicates incomplete dissociation which may require further optimization of the isolation parameters. A single cell suspension provides the best results.
Next: Measure of Viability
Tissue Tables (references, grouped by tissue type and species)
Note: We have not limited the references listed to only those papers
using Worthington enzymes. Generally speaking, the tissue dissociation enzymes
offered by Worthington can be used interchangeably for most preparations
cited.


 Place Order
Place Order 10X objective, focus on the counting chamber grid lines. Adjust the contrast as needed to clearly see both the grid and the dispersed cells.
10X objective, focus on the counting chamber grid lines. Adjust the contrast as needed to clearly see both the grid and the dispersed cells.